
- Created2024.01.30
KIMM develops technology for detecting injection of medication to prevent medical accidents related to analgesic drug infusion pump in hospitals
- KIMM develops technology for a sensor to measure low flow rate and bubbles during drug infusion
- Mass production of the world's first safe electric drug infusion pump equipped with a flow sensor is in progress
- This new technology is expected to help to prevent medical accidents resulting from excessive administration of analgesics
□ Excessive administration of analgesic drugs frequently results in medical accidents. To prevent the occurrence of these accidents, a drug infusion pump featuring a technology for safely detecting medication administration has been developed for the first time in the world.
□ The research team led by Principal Researcher Dong-kyu Lee of the Korea Institute of Machinery and Materials (President Seog-hyun Ryu, hereinafter referred to as KIMM), an institute under the jurisdiction of the Ministry of Science and ICT, has succeeded in developing the technology for customized sensor modules capable of measuring the extremely low flow rate of analgesic drug infusion pumps as well as the existence of bubbles in these pumps. A safe drug infusion pump system equipped with this sensor module is currently being developed for mass production by Unimedics (Representative Director Joo-seok Yang), one of Korea’s most renowned manufacturers of medication injectors.
□ Meanwhile, this technology was selected as the “2023 Outstanding Research Achievements by Government-funded Research Institutions” in recognition of the research achievements related thereto, including publication in an SCI journal*, filing of multiple patent applications (four (4) in Korea, one (1) in the United States), and technology transfers.
*Title of publication: “Sensitive and reliable thermal micro-flow sensor for a drug infusion” (Sensors and Actuators A: physical, date of publication: April 27, 2020)
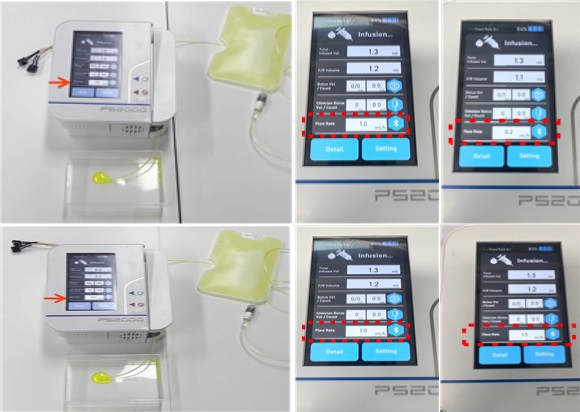
<Various flow rate detection>
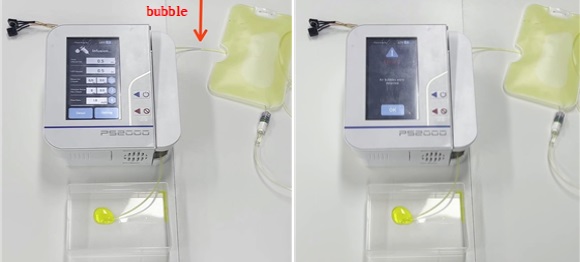
□ Additionally, in line with the revised regulations of the FDA that now require bubble sensors to be included in drug infusion pumps, the newly developed infusion pumps are equipped with temperature sensors at both ends of the assembled tube, and these sensors are capable of detecting bubbles by using the difference in heat diffusion depending on the air or liquid inside the tube. In particular, by attaching the sensor to the drug injection tube, the flow rate and bubbles can be measured non-intrusively on the outer surface of the tube. By allowing for the reuse of sensors, this can help to resolve the issues associated with the limited medical fees for medical disposables.
□ By using this technology, the research team of the KIMM secured performance equivalent to that of expensive MEMS sensors in terms of measurement sensitivity, accuracy, range, and bubble detection. The sensor has been developed as a customized module so that the ultrasonic bubble sensor within the drug infusion pump can be replaced. This sensor module is now being developed for mass production by being applied to new drug infusion pumps.
□ The conventional technology for measuring the number of drops falling into the drip chamber could not be used for analgesic drug infusion pumps that do not have drip chambers, and was relatively inaccurate (±10%) and not easily usable at low flow rates. While thermal micro-flow sensors have been developed by global companies, these sensors are mainly manufactured using expensive semiconductor processes. Additionally, there also is the economic issue associated with the fact that these sensors are all disposable due to contact between drug liquid and the sensor.
□ Therefore, an indirect flow rate measurement method involving the rotation of motors is currently being used for analgesic infusion pumps, and no device has yet been equipped with a real-time flow rate measurement technology.
□ The new technology developed by the KIMM is expected to help to prevent medical accidents resulting from excessive post-surgery administration of analgesics, which usually results from the malfunctioning of drug infusion pumps or deviations of medical consumables. It is also anticipated to enable the provision of prompt medical services by transmitting accurate information such as the data on the medication speed and dosage, and also reduce the workload of the medical staff related to drug injection management.

<Integrated drug infusion pump with flow & bubble sensor modules>
□ Principal Researcher Dong-kyu Lee of the KIMM was quoted as saying, “This is a technology for a sensor capable of simultaneously measuring extremely low flow rates and bubbles without coming into contact with the drug outside the tube and without having to apply the expensive MEMS sensor technology, simply by attaching the drug infusion tube to the sensor. It is a technology that is customized for the injection of medications.” He added, “We will make our best efforts to prevent medical accidents where overdosages of analgesics result in death during surgery or cause deaths of cancer patients, and also strive for research and development to ensure the safety of medical treatments involving drug injections and protect the safety of the public.”
□ Meanwhile, this research has been conducted with the support of the project for the “development of next-generation precise and safe analgesic injection pumps and an integrated system for drug injection management based on drug injection speed monitoring” of the Ministry of Science and ICT and the Innopolis Daedeok Development Project, and has been joined by the KIMM and Unimedics.


